Bradford Assay Troubleshooting

The Bradford protein assay is an easy and simple method for protein quantification of your protein concentration, yet may still require troubleshooting occasionally.
The Coomassie Brilliant Blue dye binds to both basic and aromatic amino acid residues, resulting in a change in color from brown to blue, and an absorbance shift. The concentration of your protein can be determined by referencing a standard protein, most commonly BSA (Bovine serum albumin).
Factors such as: temperature, wavelength, detergents and even the type of cuvettes you use can influence the measurement and provide incorrect results. Below we offer some useful tips to avoid these issues as well as solutions for when they arise.
Problem: Absorbance of the protein sample is lower than expected
- Possible Cause: Low molecular weight.
Solution: The detection limit for the Bradford assay is approximately 3000-5000 Daltons. If your protein is smaller than this, then use a different quantification method such as the BCA assay. - Possible Cause: Interfering substances.
Solution: The sample may contain interfering substances, such as detergents (Table 1). Dilute your protein sample. Be sure your standards are diluted in the same buffer.
Problem: Absorbance of the protein sample is higher than expected
- Possible Cause: The concentration of your protein is too high.
Solution: Dilute your protein sample and measure the protein concentration again. - Possible Cause: Interfering substances.
Solution: The sample may contain interfering substances, such as detergents (Table 1). Dilute your protein sample. Be sure your standards are diluted in the same buffer.
Problem: Absorbance of the standard is lower than expected
- Possible Cause: Dye reagents are too old or were improperly stored.
The Bradford Reagent has an expiry time of approximately 12 months. If older, replace it with a new one and store it at 4°C. - Possible Cause: Standard dilutions prepared incorrectly.
Follow the manufacturer's protocol for creating your protein standard dilutions. - Possible Cause: Dye reagent may be too cold.
Solution: Bring the Bradford Reagent to room temperature before beginning the assay. - Possible Cause: Absorbance measured at incorrect wavelength.
Solution: Measure the absorbance at 595 nm.
Problem: Samples are dark blue
- Possible Cause: High alkaline concentrations.
Solution: This raises the pH above the Bradford reagent’s limit. Dilute or dialyze your sample.
Problem: Precipitates in the sample
- Possible Cause: Detergents in your protein buffer.
Solution: Dialyze your protein sample or dilute the sample to reduce the level of detergents.
Problem: The protein sample contains interfering substances
One of the major problems encountered when performing a Bradford assay is the presence of interfering substances in the buffer. Table 1 summarizes a list of the most commonly used substances and their compatible concentrations. For detailed information, please refer to the manufacturers’ protocol.
Table 1: Compatible substance concentrations in Bradford assay (modified after [1] Quick Start Bradford Protein Assay – Instruction Manual, #4110065, Bio-Rad; [2] Protein assay compatibility table, Tech tip #68, (2012))
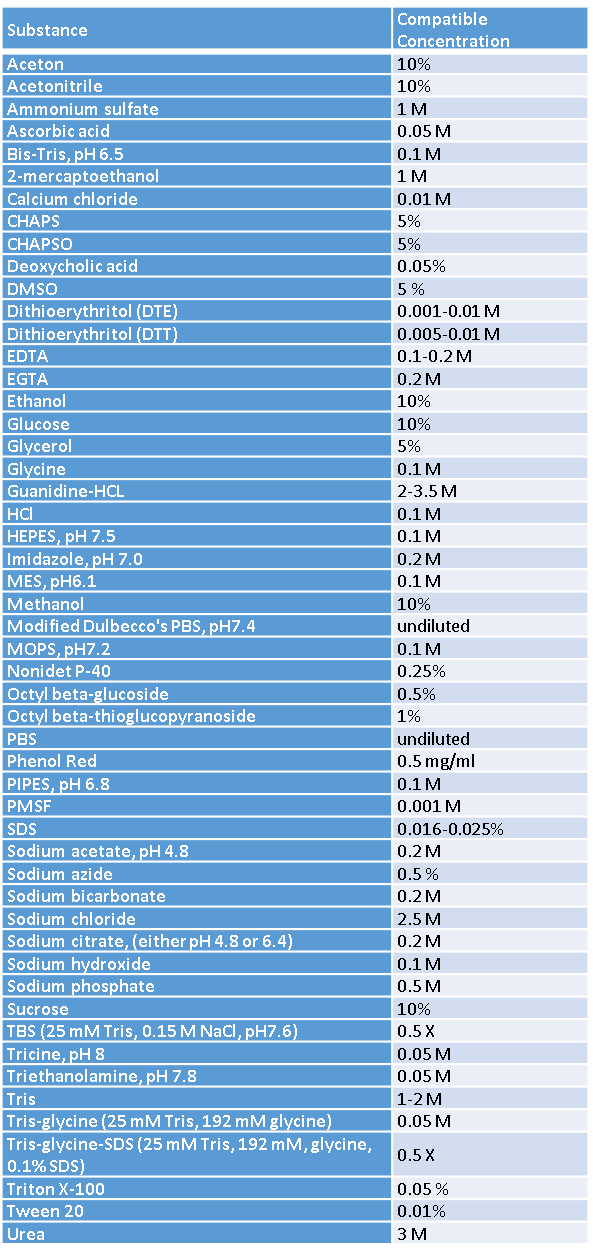
Note: This is not a comprehensive list of compatible substances and their concentrations. There are many substances that can affect proteins in different ways. Reagents may also change the pH of the assay and interfere with the Bradford assay.
If the substance you are using is not listed above or remains at an incompatible
concentration, then see below:
- Possible Cause: Concentration of interfering substance is incompatible with the Bradford assay.
Solution: Dilute the sample to the point of no interference if your protein concentration is high enough, or remove the substance by dialysis. - Possible Cause: Substance is not in the reagent compatibility list.
Solution: Run two standard curves, one with your protein in buffer, one with your protein in water and plot a graph of protein concentration versus absorbance. If the buffer is not interfering with your measurement, then the two standard curves will have the same slope.
Technical tips
- The dye can react with quartz cuvettes; use glass or plastic cuvettes instead,
- Ensure glassware is clean before use to prevent interference from contaminants,
- Bradford reagent should be used at room temperature.